
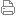
9. (a) Figure below shows the symbol representing an atom of sodium
nucleon no. : 23
proton no. : 11
symbol of element: Na
Based on the information given, elaborate the structure of sodium atom. your answer should include the diagram of electron arrangement and number of subatomic particles in the sodium atom. (4 marks)
(b) Organic compound X is a solid at room temperature and pressure. The melting point of X is less than 100°C.
(i) Describe a laboratory experiment to determine the freezing point of solid X. your answer should consist of following.
--> List of materials and apparatus required
--> Diagram showing the set-up of apparatus
--> Procedure of the experiment
--> Tabulation of data
--> Interpretation of data (10 marks)
(ii) Describe the changes occurred in the arrangement of molecules, attractive forces between the molecules and the energy content of compound X when it is cooled from 100°C to room temperature. (6 marks)
10. (a) (i) What is a chemical formula? (1 mark)
(ii) State the qualitative and quantitative information that a chemical equation can provide. (3 marks)
(b) (i) Name a metal which is less reactive than hydrogen in the reactivity series. (1 mark)
(ii) Describe a laboratory experiment to determine the empirical formula of the oxide of the metal named in (b)(i) above. (10 marks)
(c) Nowadays, most of the cars are equipped with airbags that sodium azide, NaN3. Sodium azide decomposes quickly when heated to produce nitrogen gas and sodium. The gas prodeced will expand the air bag.
Calculate the mass of sodium azide required to produce just enough nitrogen gas to inflate an air bag of volume 35 dm3 at room conditions. (relative atomic mass: N,14; Na, 23; Molar volume of gas at room conditions = 24 dm3 mol-1) (5 marks)









 那第10题的是哪一个experiment的?
那第10题的是哪一个experiment的?